Breast Tissue Expanders
Eileen, Actual Sientra Patient
BreastTissue Expanders
Eileen, Actual Sientra Patient
See State-of-the-Art Breast Tissue Expander Options Designed for Comfort and Compassion
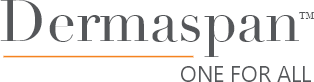
Soft, Refined Design Allows for Gentle and More Comfortable Expansion
- Soft, pliable shell results in less friction upon insertion
- Designed without ridges or rings for improved comfort
- Port location is designed to aid in predictable, consistent expansion
*as compared to Dermaspan with 2 tabs
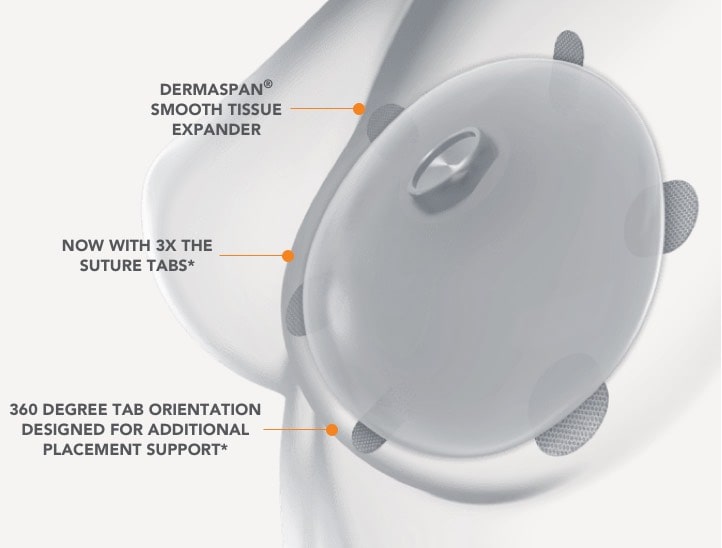
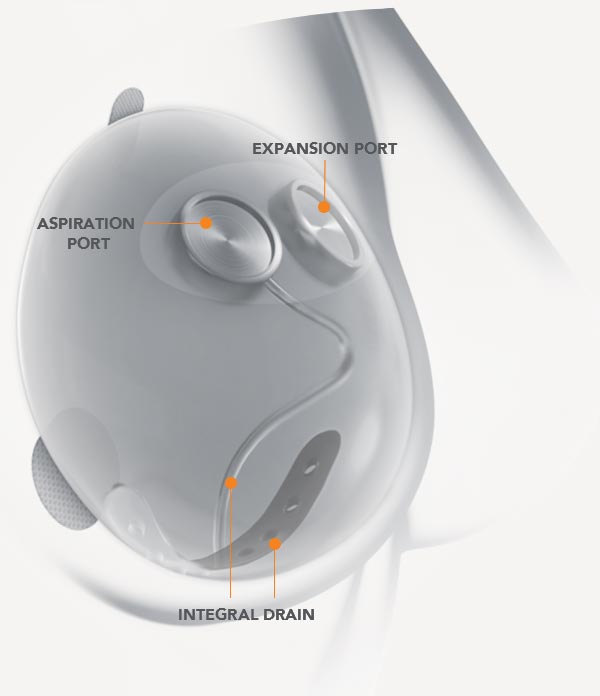

Revolutionary Dual-Port Design and Integral Drain Allow for Less Invasive – Non-Surgical – Draining of Serous Fluid
- Designed to mitigate key risks associated with breast expansion and ultimately reduce reoperation rates
- Only tissue expander with an integral drain providing direct access to the periproshetic space where fluid can accumulate
- Allows for diagnostic fluid sampling to enable a faster treatment response
*as compared to Dermaspan with 2 tabs
The unique feature of the AlloX2 provides surgeons easy access to the periprosthetic space without altering any of the other characteristics of a tissue expander. While one does not plan to experience a postoperative complication, a valid question in light of the availability of the AlloX2 is why one would forego utilizing a device that facilitates treatment thereof.
– ARASH MOMENI, MD
Board-certified plastic and reconstructive surgeon | Palo Alto, CA
See AlloX2 Reduce the Financial Risks Associated with Breast Reconstruction
Managing the Risks of Post-Operative Fluid Accumulation
Obesity (BMI >30) and use of an Acellular Dermal Matrix (ADM) have been identified as major risk factors for seroma4
- ~40% of adult patients in the US are obese5
- An ADM is used in over 60% of breast reconstruction cases6
A seroma increases the risk of major infection in breast reconstruction patients
- Risk of infection is 4x higher in patients who develop a seroma4
- Risk of expander loss caused by infection is 6.7x higher in patients who develop a seroma4
Clinically relevant infections with poor salvage rates occurred in nearly 1/5 of seroma patients (18.8%)4
With current standard of care, 78% of infections require explantation4
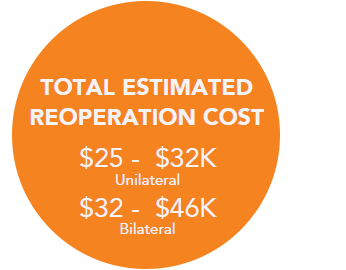
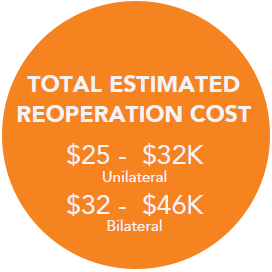
Re-Operation Costs Can Run Upwards of +$20K,7 Which May Not Be Covered By Insurance if Within 30 Days of Initial Surgery8






*ADM currently not approved for use in breast surgery. Please refer to product labeling for more information.


See Clinical Results
AlloX2 Improves Salvage Rates & Clinical Outcomes10
Retrospective review of 112 consecutive patients
- 63 patients (53.6%) Mentor Artoura
- 49 patients (43.7%) AlloX2
- 173 breasts with smooth tissue expanders (2016-2017)
AlloX2 Stats:
- 8% seroma rate
- All successfully drained in clinic through AlloX2
- 1 patient’s aspiration tested positive for MRSA which allowed immediate antibiotic intervention and salvage of reconstruction without explant
100% Surgeon Satisfaction Using AlloX211
In a clinical study with 40 primary reconstructive patients, the AlloX2 was deemed successful in treating seromas and should be considered a tool for noninvasive treatment of common complications of tissue expander-based breast reconstruction.


